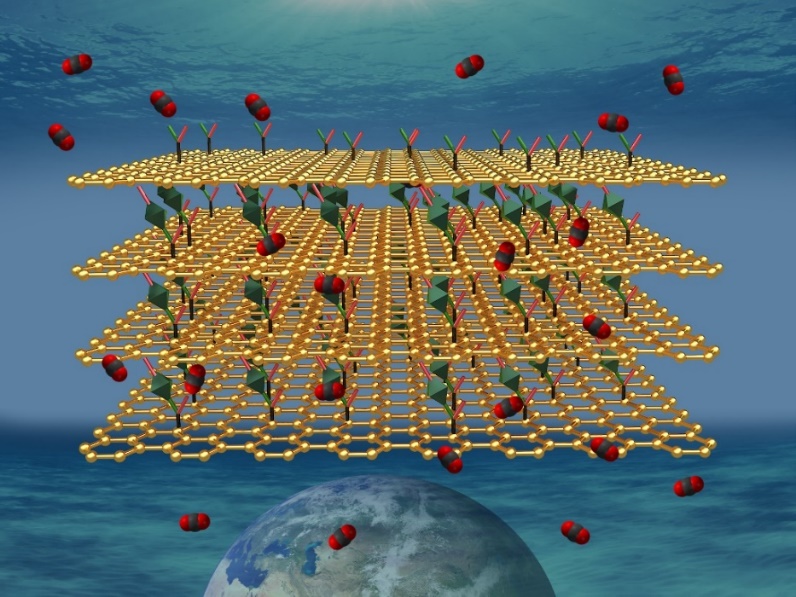
Detectors with a rapid detection time, low detection limits, high selectivity and sensitivity towards CO2 gas are important for health and safety applications, for example in the food industry or for monitoring air quality indoor and in mines. An international team of scientists, including CATRIN representatives, has developed hybrids of graphene and metal-organic frameworks (MOFs) effective as chemoresistive gas sensors.
“One of the most critical challenges in the development of CO2 gas sensors is the generation of active sites for adsorption/desorption, where CO2 binding can cause signals, e.g., changes of electrical parameters,” says one of the main authors of the work, Dr. Kolleboyina.
The scientists created suitable CO2 binding sites in a metal-organic framework (MOF) material. MOFs are crystalline porous materials made of a regular network of ions linked via organic linkers. However, MOFs are not conductive and may suffer from poor stability. In order to introduce conductivity to MOFs, the team at RCPTM led by Michal Otyepka and Aristeides Bakandritsos prepared a nanocomposite with graphene acid developed here. “Graphene acid is a conductive graphene derivative synthetized via the chemistry of fluorographene, which we developed under the support of ERC. It also works as a nice templating agent suitable for hybridization with MOFs”, added Michal Otyepka.
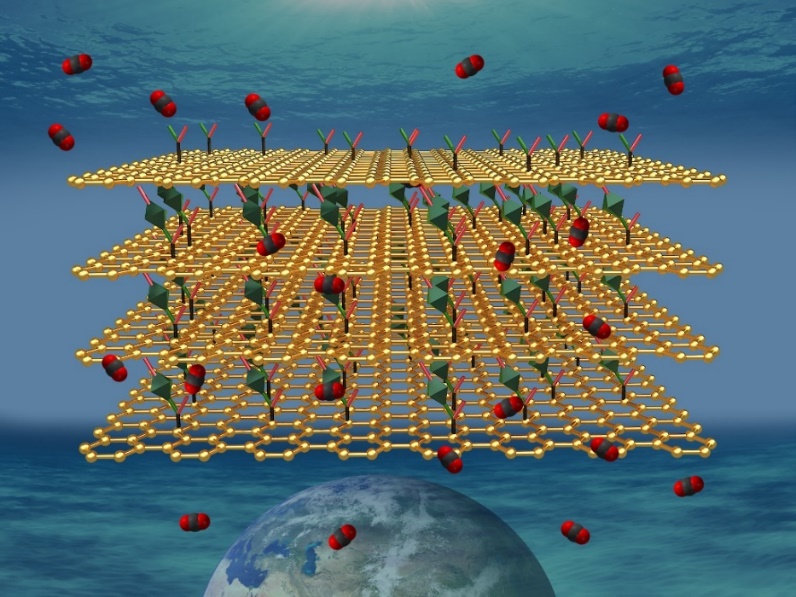
The prepared material fulfilled the critical parameters for efficient chemiresistive CO2 sensing as it has high-surface area, hierarchical porosity, suitable interaction sites for CO2 binding, significant chemical and thermal stability, and is conductive. The hybrid worked as a CO2 efficient chemiresistive gas sensor with a quick recovery time—the fastest among other MOF-based chemiresistive sensors. The research results were published in the Journal of Materials Chemistry A.